Martempering(Previous)-GROUP:Heat treatment
method
Martempering (marquenching) is a modified quenching procedure used for steels to minimize distortion and cracking that may develop during uneven cooling of the heat-treated material. The martempering process consists of (1) austenitizing the steel, (2) quenching it in hot oil or molten salt at a temperature just slightly above (or slightly below) the Ms temperature, (3) holding the steel in the quenching medium until the temperature is uniform throughout and stopping this isothermal treatment before the austenite-to-bainite transormation begins, and (4) cooling at a moderate rate to room temperature to prevent large temperature differences. The steel is subsequently tempered by the conventionel treatment.
The structure of the martempered steel is martensite and that of the martempered (marquenched) steel which is subsequently tempered is tempered martensite.
(Smith W. F., Foundations of materials science and engineering, Ed. 2nd, 449,450)
Martempering (marquenching) is a modified quenching procedure used for steels to minimize distortion and cracking that may develop during uneven cooling of the heat-treated material. The martempering process consists of (1) austenitizing the steel, (2) quenching it in hot oil or molten salt at a temperature just slightly above (or slightly below) the Ms temperature, (3) holding the steel in the quenching medium until the temperature is uniform throughout and stopping this isothermal treatment before the austenite-to-bainite transormation begins, and (4) cooling at a moderate rate to room temperature to prevent large temperature differences. The steel is subsequently tempered by the conventionel treatment.
The structure of the martempered steel is martensite and that of the martempered (marquenched) steel which is subsequently tempered is tempered martensite.
(Smith W. F., Foundations of materials science and engineering, Ed. 2nd, 449,450)
Martempering(New)
Martempering involves heating the
steel 10 the austenitizing temperature, followed by quenching in a constant
temperature bath maintained above M, point. The usual temperature of the bath
lies between I80°C and 250°C. Steel is held in the bath (ill temperature
throughout the section becomes uniform and is equal to the bath temperature. As
soon as this temperature is attained, steel is withdrawn and cooled in air. The
cooling rate should be sufficiently high and holding time considerably short to
prevent transformation of austenite to pearlite or to bainite. Martensitic is
formed in the second stage, namely, during cooling in air. The cooling sequence
for martempering superimposed on TTT diagram is shown in Figure 5.9.
Martempering results in minimum internal stresses, reduced tendency towards
distortion and cracking and improved mechanical properties as compared to
conventional quenching and tempering treatment. The resultant microstructure of
martempered steel is martensite. In order to improve properties, marempered
steels are generally tempered.
Since the success of the process
depends on the formation of martensite. alloy steels are best suited for the
process. Almost all alloying elements, except cobalt, increase hardenability.
Any steel, which can be hardened by oil quenching, can be martempered
successfully. Smaller sections of these steels which can only be hardened by
water quenching can also be employed for this process. A large number of steels
satisfy these requirements. Another advantage of using alloy steels is that
alloying elements increase incubation period. This results in availability of
sufficient holding time.
(T.V. Rajan, C.P. Sharma and Ashok Sharma, Heat Treatment Principles and Techniques,page
105)
New
definition is more understandable than previous one. It is better.
Diffusion Coating (Previous)-GROUP:Coating method
Diffusion Coating processes have been applied for many
years to improve the enviromental residence of a base alloy by enriching the
surface in Cr, Al or Si. Diffusion Coatings be applied to hot-gas components
using several techniques, including pack cementation, slurry cementation, and
metallising. Fludised bed techniques can also be used to deposit diffusion
coatings on a laboratory scale.
In the pack cementation process, components to be
coated and buried in a pack contained in a sealed retort. The exact process
cycle, time, and temperature are depended on the required coating, coating
thickness and subsequent substrate heat treatment. The pack contains three
elements 1-) a donor alloy that releases solute material at a known rate and
hence determines the pack activity 2-) a halide activator that dissociates the
process cycle and acts to transport solute material from the back to the
component to be coated, and 3-) an inert oxide diluent prevent pack - sintering
(Physical Metallurgy Vol. 1 , Cahn W.R., Haasen P.,
pages #1345-1346)
Diffusion Coating (New)
Diffusion
coatings (Goward and Seigle, 1994; Goward, 1998) consist of a substrate alloy
surface layer enriched with the oxide scale formers Al, Cr, Si, or their
combination to a depth of 10 to l00 µm. These elements combine with the primary
constituents of the substrate alloy to form intermetallics with significant
levels of the oxide scale formers. For example, in Ni base superalloys, surface
enrichment with aluminum forms nickel aluminide, NiAl (the β phase in the Ni-AI
system), which is the predominant constituent of the coating. The substrate
alloy participates in the formation of diffusion coatings. For oxidation
protection, the diffusion coatings of choice are the aluminides, which form a
protective alumina scale on high-temperature exposure in air. For protection
against hot corrosion, incorporation of platinum in the aluminide, chromizing,
and siliconizing are more beneficial. Because the β phase field in the Ni-AI system is quite
broad in composition range, the Al content in diffusion aluminides can vary
within a wide range with typical aluminides having Al <30wt %. Higher Al
content results in hyper stoichiometric composition. Such aluminides exhibit a
bluish tint and are called '"blue beta". Lower aluminum content, on
the other hand, results in the hypo-stoichiometric composition. Compositions of
diffusion coatings based on Cr and Si also may vary over a wide range. These
coatings can be applied to components of complex shapes. The coating process
involves exposure to high temperature. Additional post coating heat treatment
may be required to restore substrate properties such as creep and fatigue
strength. As the heat-treatment temperature seldom exceeds 1100°C (2012°F),
which can be done in traditional furnaces, the coating equipments and facilities
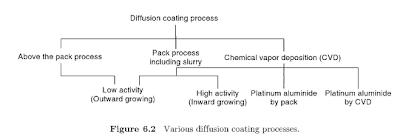
do not require large capital investments. Diffusion coatings, which are
essentially a type of surface enrichment in which vapors are deposited, can be
produced by one of several methods described in Fig. G.2. The basic process
consists of the following steps:
• Generation of Al-, Cr-, or
Si-containing vapors
• Transport of the vapors to the
component surface
• Reaction of the vapors with the
substrate alloy followed by associated diffusion processes within the alloy
• Additional heat treatments are
necessary to achieve desired coating composition and coating as well as substrate
properties
• The microstructure, Al, Cr, or Si
activity in the coating and coating thickness depend on the substrate alloy,
the process parameters including temperature and subsequent heat treatment.
(Sudhangshu Bose,
High temperature coatings,
pages 73-74)
New definition is more
understandable and obvious than previous one. It is better one.
RTV silicone molds (Previous)-GROUP:Rapid prototyping method
One of the most widely used approaches to rapid tooling involves making room-temperature vulcanizing (RTV) silicone molds from a pattern made on any one of the RP/RM processes. The RTV mold may be fabricated in two pieces, or it may be a one-piece cut mold. Regardless of how the RTV mold is made, polyurethane or other two-part resins are then cast into the mold to produce a plastic part. The urethane may include dyes and/or fillers to modify its appearance and material properties. Common fillers include glass beads to reduce part weight and to reduce the volume (and cost) of the resin being used. Chopped fiberglass strands sometimes are added to increase part strength. Metal powders such as bronze are also added at times to make the part look and feel like a metal part. Provided the powder loading is sufficiently high, it is possible to buff the parts in order to achieve a somewhat shiny metallic look. Depending on the resin being cast, the workpiece geometry, and any fillers/additives, the typical RTV mold usually is good for approximately 30 to 50 castings.
(Ronal A. Wash, Denis Cormier , McGrawHill Machining and Metalworking Handbook , page 721)
One of the most widely used approaches to rapid tooling involves making room-temperature vulcanizing (RTV) silicone molds from a pattern made on any one of the RP/RM processes. The RTV mold may be fabricated in two pieces, or it may be a one-piece cut mold. Regardless of how the RTV mold is made, polyurethane or other two-part resins are then cast into the mold to produce a plastic part. The urethane may include dyes and/or fillers to modify its appearance and material properties. Common fillers include glass beads to reduce part weight and to reduce the volume (and cost) of the resin being used. Chopped fiberglass strands sometimes are added to increase part strength. Metal powders such as bronze are also added at times to make the part look and feel like a metal part. Provided the powder loading is sufficiently high, it is possible to buff the parts in order to achieve a somewhat shiny metallic look. Depending on the resin being cast, the workpiece geometry, and any fillers/additives, the typical RTV mold usually is good for approximately 30 to 50 castings.
(Ronal A. Wash, Denis Cormier , McGrawHill Machining and Metalworking Handbook , page 721)
RTV silicone molds (New)
RTV silicone rubber molds One
of the most popular tooling applications for RT (rapid tooling) is the
production of room temperature vulcanizing ( RTV) silicone rubber molds. This
room temperature system is the vulcanization or curing at room temperature by
chemical reaction, made up of two-part components of silicones and other
elastomers/rubbers. RTV are used to withstand temperatures as high as 290°C
(550°F) and as low as -160°C (-250°F) without losing their
strength. Their rapid curing makes them useful in different applications such
as prototypes or prototype molds. Silicone is a versatile material that can be molded around a master
pattern to produce a cavity with the advent of RT techniques; master patterns
are often an RP model. Silicone rubber molds are also used to produce urethane, epoxy, and etc.
prototypes.
Silicone
rubber mold provides fast, relatively inexpensive molds, excellent product
cosmetics, and the option of using multiple materials. The process is suitable
for small or large products. Due to material and labor costs individual product
prices are relatively high. Even with this type of potential limitation,
silicone rubber tooling is used as a production process.
To produce
a rubber mold consists of making a master pattern, finishing the pattern lo the
desired appearance and casting RTV silicone rubber around the pattern to form the
mold. With transparent or other material the model is suspended within a box
and silicone rubber is poured to fully surround the model. After the silicone
rubber has solidified, the parting line is cut with a scalpel and the model
removed, leaving the required cavity.
When
polyurethane (and other plastics such as acrylic) is poured into the silicone
rubber cavity it is usually under vacuum lo avoid air bubbles in the molded
component. The silicone rubber tool will generally produce about 20
polyurethane products before it begins to deteriorate. This will depend on the
amount of detail in the tool and the type of polyurethane being molded.
Flexible polyurethanes require longer post cure times within the mold, which is
placed in the oven a 65°C (149 °F). This prolonged contact dries out the
surface of the silicone rubber and renders it more brittle. Once this occurs,
line detail on the inner surface of the mold starts to disintegrate.
(Dominick
V. Rosato, Plastics engineering, manufacturing & data
handbook, pages 1425-1426)
New
definition is more understandable and obvious than previous one. It is better
one.
Double tempering(Previous)-GROUP: Heat treatment
A treatment in which a quench-hardened ferrous metal
is subjected to two complete tempering cycles, usually at substantially the
same temperature, for the purpose of ensuring completion of the tempering
reaction and promoting stability of the resulting microstructure.
(Principles of the Heat Treatment of plain carbon and
low alloy steels, Brooks C.R., Page #472)
Double
Tempering (New)
In tempering high-speed steel tools, it is
common practice to repeat the tempering operation or "double temper"
the steel. This is done by heating the steel to the tempering temperature (say
1050 ᵒF) and holding it at that temperature for two hours. It is then cooled
to room temperature, re-heated to 1050 ᵒF for another two-hour period,
and again cooled to room temperature. After the first tempering operation, some
un-tempered martensite remains in the steel. This martensite is not only
tempered by a second tempering operation but is relieved of internal stresses,
thus improving the steel for service conditions.
(Franklin Day
Jones, Machine shop training course, page 489)
New definition
is more specific and obvious than previous one. It is better.
Cold
Treatment (Previous)-GROUP: Heat Treatment Method
Strength can be improved in hardened steels containing retained austenite by a process known as sub-zero treatment or cold treatment. Retained austenite is converted into martensite by this treatment. This conversion of retained austenite into martensite results in increased hardness, wear resistance and dimensional stability of steel.
The process consists of cooling steel to subzero temperature which should be lower than the Mf temperature of the steel. Mf temperature for most steels lies between -30 C and -70 C. During the process, considerable amount of internal stresses are developed in the steel, and hence tempering is done immediately after the treatment
(T. V. Rajan, C. P. Sharma, Ashok Sharma, Heat Treatment: Principles and Techniques, page 121)
Strength can be improved in hardened steels containing retained austenite by a process known as sub-zero treatment or cold treatment. Retained austenite is converted into martensite by this treatment. This conversion of retained austenite into martensite results in increased hardness, wear resistance and dimensional stability of steel.
The process consists of cooling steel to subzero temperature which should be lower than the Mf temperature of the steel. Mf temperature for most steels lies between -30 C and -70 C. During the process, considerable amount of internal stresses are developed in the steel, and hence tempering is done immediately after the treatment
(T. V. Rajan, C. P. Sharma, Ashok Sharma, Heat Treatment: Principles and Techniques, page 121)
Cold Treatment (New)
Cooling to sub-zero temperatures, either with dry ice (solid CO2;
to -84 ᵒC or -120 ᵒF) or liquid nitrogen (-190 ᵒC or -310 ᵒF has been shown to
improve the properties of some metal products. It can complete the austenitic
lo martensite transformation when the martensite finish temperature is low, as
with high-carbon and highly alloyed steels, thereby increasing strength and
hardness. Performance improvements have also been noted for a number of
nonferrous metals, and the underlying reason appears to be the relaxation or
removal of the unfavorable residual stresses. By slow cooling lo such extreme
temperatures, and then slowly healing hack lo room temperature, the parts
undergo a significant amount of uniform thermal contraction, and this is often
sufficient to induce the small amounts of deformation necessary to relax the
residual stresses. Numerous parts, such as race-car engine blocks and brake
rotors, exhibit reduced wear, longer lifetime, and enhanced performance after
cryogenic processing. Thermal shock is avoided by slowly feeding the liquid
nitrogen and cooling the part in the evaporated gas.
(E. Paul DeGarmo,J. T. Black,Ronald A. Kohser, Degarmo's
Materials and Processes in Manufacturing, pages 142-143)
New definition is more obvious and general
than previous one. It is better one.

No comments:
Post a Comment