PHOTOLITHOGRAPHY (OPTICAL LITHOGRAPHY)
group: material process
Old
definition:
Optical
Lithography(also called photolithography) uses light as the writing material.
The drawn(greased) and undrawn(moistened) areas on the limestone in lithography
become bright and dark regions on a reticle or phptmask, the template of
optical lithography. Just as greased ink discriminately deposits on the
limestone, light passes only through the clear opening the mask. The
transmitted energy is recorded on a light-sensitive medium called the
photoresist.
(Alfred Kwok- Kit
Wong, Resolution Enhancement Techniques In Optical Lithograpy,p.2)
New definition:
An often-used
technique for device fabrication is photolithographic masking. Here, a film of
photoresist is applied to the substrate, and the photoresist is exposed to
light through a photolithographic mask. After exposure, the photoresist is
developed, which transfers the desired pattern to the photoresist (Fig. 6.3).
When the substrate is subjected to a chemical treatment, the photoresist
protects the surface, and thus the pattern on the mask is transferred to the
substrate. The photoresist is removed by stripping, which is essentially
dissolution of the photoresist in a nonuniform but fast way. Acetone is usually
used to strip resist, but in some cases a special stripper must be used. The
photoresist manufacturer generally makes such a stripper available.
Photoresists can be any of various photosensitive polymers. These polymers can
be applied by different techniques such as spinning or spraying. In spinning,
the thickness of the film can be expressed by this empirical expression:
Where t coating is the
thickness of the coating, K is a
proportionality constant, r is the
kinematic viscosity (mm2 s-1), and w is the number of revolutions per minute. The polymers are
sensitive to light of particular wavelengths, and when exposed to these the
chemical structure of the photoresist changes. Usually they are sensitive to UV
light, with the i-line of mercury (365 nm) being particularly popular. After
exposure to light, the photoresist is developed by using a particular chemical
solution. Some photoresists become more soluble in a developer after exposure,
some become less soluble. The photoresists that become more soluble are called
positive tone photoresists (Fig. (6.3); an example is AZ4562 photoresist. The
other type of photoresist becomes less soluble after light exposure, due to
cross linking of the polymer, and is called negative tone photoresist; an
example is SU-8.
(Oliver
Geschke,Henning Klank,Pieter Telleman,
Microsystem Engineering of Lab-On-A-Chip Devices, pg. 122)
My definition makes it easy to understand about this method by
given formulations and layout of process.
BLACK ‘ORLON’
group:
material
Old
definition:
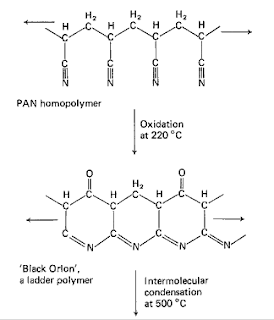
Black Orlon is a
ladder polymer obtained by regulated pyrolysis of polyacrylonitrile. The
structure of black orlon contains H,C and N atoms. Fibres of black orlon are
highly tough and find applications in fibre reinforced plastics.
(A.K. Bhargava,
Engineering Materials: Polymers Ceramics And Composites, 200, page 118)
New definition:
For some time it
has been known that PAN fibres blacken on heating in air at 220 0C
and that this 'black Orion' is remarkably flame-proof and stable. Such
stabilization by oxidation permits the formation of oriented ladder polymer
whilst reducing the intensity of the exothermic peak, which would otherwise
build up if the oxidation process were to be omitted. After stabilization, the
modified ladder molecules have a high enough glass transition temperature to
retain any preferred orientation imposed in the original polymer, even subsequent
to a carbonization process.
The proposed
mechanism of carbonization is illustrated also in fig. 9. The ladder molecules
coalesce progressively to form the ribbons characteristic of all polymeric
carbons. It should be stressed that the structures drawn in fig. 9 are
essentially related to isotactic homopolymers, whereas the fibres used to make
high stiffness, high tenacity carbon fibres are atactic copolymers. This makes
the true mechanism difficult to interpret.
(G. M. Jenkins,K.
Kawamura, Polymeric Carbons,
pg.31-33)
Old definition is also good because of being simple and easy to
understand but against my definition is more satisfactory to observe the
structure.
VGCF (VAPOR
GROWN CARBON FIBERS)
group:
material
Old
definition:
VGCFs comprise a
large family of filamentous nanocarbons.Thwy can be distinguished in terms of
arrangement of the graphane layers in their molecular scale structure.They can
be plate-like with near paralell graphane layers that are approximately
perpendicular to the fiber axis or they can have the 'fish-bone' microstructure
with stacked cones of graphane planes.(Functional Fillers For Plastics,Marino
Xanhtos,p:194)
New definition:
Carbon fibers
produced by direct growth from the vapor phase, i.e., vapor grown carbon fibers
(VGCF)is an another variety of reinforcement with high mechanical and thermal
properties and significant commercial potential. These are also called catalytic
chemical vapor deposition (CCVD) carbon fibers. These fibers arc produced by
catalytic decomposition of a hydrocarbon such as benzene, methane, or propane
at 1000-I 500o C in the presence of a transition metal (Fe, Ni, or
Co) or metallo-organics such as ferrocene. (C 5H5)2,Fe
(Tibbetts ei at 1987). The catalyst plays a vital role in the growth of VGCFs.
The carbon fiber yield is enhanced if a catalyst with a small but broader
distribution of particle sizes is used. VGCFs are produced in short lengths (50-70
mm) and small diameter (0.5-2 pm). Benzene, or other hydrocarbons which
generate benzene during decomposition, are preferred as a precursor for a
higher rate of production. These fibers have also been produced from low cost
sources such as linz-donawitz converter gas and coal-derived hydrocarbons.
These fibers possess very high mechanical properties but have a large scatter
in the values.
The structure, and
thus the mechanical properties of VGCF, are independent of the precursor gas
source employed, but are extremely dependent on processing parameters such as
temperature of growth, type and distribution of catalyst, etc. The aspect ratio
of VGCF is found to be dependent on catalyst to hydrocarbon flow ratio. Low
temperature deposition (<9000C) results in vermicular filaments,
while high temperatures (1500-25000C) favor the growth of long,
straight filaments. Since VGCF are produced in a one-step process, these
possess significant commercial potential as low cost carbon fibers.
Based on electron microscopic
studies on the structure of the fibers, the growth process has been proposed
consisting of two parts (Endo et al. 1977). In the primary process a thin tube
of carbon is formed by catalytic growth on a catalyst particle. This is followed
by secondary growth onto the first tube. VGCF consist of turbostratic carbon
layers parallel to the fiber axis arranged in annular concentric sheets like
rings in a tree trunk. The core is more perfectly ordered while the secondary
sheets may contain some defects. VGCF are graphitizable carbons, and when heat
treated to more than 2500 0C, develop a well-orianted graphite
structure and increase in density and Young’s modulus.
(Andreas Mortensen, Concise Encyclopedia of Composite
Materials, pg. 93 )
My definition is so good against the older definition because
the previous one is so poor to explain the process.
CHEMICAL
SYNTHESIS
group:
material chemistry
Old
definition:
Chemical synthesis
is uniquelly positioned at the heart of chemistry, the central science, and its
impact on our lives and society is all pervasive. For instance, many of todays
medicines are synthetic and many of tomorrows will be conceived and produced by
synthetic chemist. To the field of synthetic chemistry belongs an array of
responsibilities which are crucial for the future of mankind, not only with
regard to the health, material and economic needs of our society, but also for
the attainment of understanding of matter, chemical change and life at the
highest level of which the human minds is capable.
(Chemistry 1981-1990,
B.G. Malmström, p.686)
New definition:
A century and a half later
Crnforth defined chemical synthesis as "international construction of molecules
by chemical means". The period from the second half of the 19th century
through the first half of the 20th century witnessed three primary pursuits in
the field of organic chemistry: (I) the elucidation of the structures of
natural products, (2) the investigation of basic reactions, and (3) the
preparation of new chemical substances (Fig. 1.1-1). These activities continue
to this day simplified by modern technology. Organic chemists pursue new activities
as %sell, and synthetic organic chemistry is now associated with biology,
medicinal chemistry, and materials sciences.
Any chemical
synthesis can be resolved into three basic processes as shown in Table I. The
information associated with each process must be captured and stored in an
automated procedure. The design process is a melding of target selection with
synthetic methodology. For a traditional synthesis, the potential targets are
only limited by the chemist's skill. Because the glassware used for traditional
syntheses is of modular design and can be built into a large number of
configurations, the reactor design generally does not impose any restrictions
on the reactions that can be run. Thus the chemist can select any method for
synthesis of the desired targets. The commercial availability of chemical
building blocks becomes a major factor in library design. Due to the long lead
times for custom-synthesized building blocks, the synthesis of compound
libraries for the drug discovery are often restricted to commercially available
starting materials. Availability of building blocks also impact the selection
of the chemistry used to synthesize the final products. The synthetic
methodologies are restricted to those chemical reactions that use the available
building blocks. The synthetic transformations must also be selected that
provide the best chance of success (e.g., high yield limited reaction steps,
solution or solid phase techniques, easy to handle reagents, common solvents,
etc.).
(Tomás Hudlicky,Josephine
W. Reed, The Way of Synthesis: Evolution
of Design and Methods for Natural Products, pg. 3-4)
(By Swartz, Analytical Techniques in Combinatorial
Chemistry, pg.177)
My definition is richer for an engineer to understand how and
why chemical synthesis used in our lifes.
CHEMICAL VAPOR DEPOSITION
group:
material process
Old
definition:
Chemical vapor
deposition (CVD) is a widely used materials- processing technology. The
majority of its applications involve applying solid thin-film coating to
surfaces, but it is also used to produce high-purity bulk materials and
powders, as well as fabrikating composite materials via infiltration
techniques. It has been used to deposit a very wide range of materials. The
majority of the elements in the periodic table have been deposited by CVD
techniques, some in the form of the pure elements, but more often combined to
form compounds.
(Jong-Hee Park,T.
S. Sudarshan, Chemical vapor deposition, p. 1)
New definition:
Thermal CVD (or
vapor plating) is the deposition of atoms or molecules by the high temperature
reduction or decomposition of a chemical vapor precursor species, which
contains the material to be deposited. Reduction is normally accomplished by
hydrogen at an elevated temperature. Decomposition is accomplished by thermal
activation. The deposited material may react with other gaseous species in the
system to give compounds (e.g. oxides, nitrides). Chemical vapor deposition
processing is generally accompanied by volatile reaction byproducts and unused
precursor species. Chemical vapor deposition has numerous other names and
adjectives associated with it such as vapor phase epitaxy (VPE) when CVD is
used to deposit single crystal films, metalorganic CVD (MOCVD) when the
precursor gas is a metalorganic species, plasma-enhanced CVD (PECVD) when a
plasma is used to induce or enhance decomposition and reaction, and low
pressure CVD (LPCVD) when the pressure is less than ambient. Plasmas may be
used in CVD reactors to "activate" and partially decompose the
precursor species. This allows deposition at a temperature lower than thermal
CVD and the process is called plasma-enhanced CVD (PECVD) or plasma-assisted
CVD (PACVD). The plasmas are typically generated by radio frequency (rf)
techniques. Figure 1.2 shows a parallel plate CVD reactor that uses rf power to
generate the plasma. This type of PECVD reactor is in common use in the
semiconductor industry to deposit silicon nitride (Si3N4) and phosphosilicate
glass (PSG), encapsulating layers a few microns thick with deposition rates of
5-100 nm/min. at low pressures, concurrent energetic particle bombardment
during deposition can affect the properties of filns deposited by PECVD.
CVD Diamond Cutting
Tools
Chemical vapor
deposition processes now permit the economical manufacture of large sizes and
commercial quantities of synthetic diamond at temperatures less than 1000 0C(1830 °F) at relatively low
pressures (<100 kPa, or 1 atm). CVD diamond has as low a coefficient of
friction as Teflon, is as hard as natural diamond, and exhibits thermal
conductivity four to five times that of copper. Tools coated with diamond can
machine a wide variety of nonferrous materials. The coating exhibits high
lubricity, generates low cutting forces. wears slowly, and does not heat-distort
the workpiece. The properties of single-crystal diamond, CVD diamond, and PCD
arc compared in Table 9.
The CVD process can
he defined as the deposition of a solid on a heated surface via a chemical
reaction from the vapor or gas phase. It belongs to the class of vapor-transport
processes that arc atomistic in nature, that is, the deposition species are
atoms or molecules, or a combination thereof.
The CVD of diamond
requires the presence of atomic hydrogen, which selectively removes graphite
and activates and stabilities the diamond structure. To dissociate hydrogen
requites a high-energy source. In addition to the need for atomic hydrogen,
other factors, such as energy output and the presence of oxygen, have been
shown to be important as well. Although the deposition mechanism associated
with CVD processing of diamond is complex, the basic reaction involves the decomposition
of a hydrocarbon, such as methane:
CH4→C(diamond)+2H2(g)
The reaction can be
activated by microwave plasma or direct current (dc) plasma arc.
Applications
CVD and PCD diamond
can be used in many of the same applications, but PCD is more suited to
roughing and to machining applications and materials that require high fracture
toughness of the tool. CVD diamond excels at finishing, semi-finishing, and continuous
turning applications because of its superior wear resistance, and its hardness
allows it to produce more precisely machined parts. Materials commonly machined
by CVD diamond include high-silicon content aluminum casting alloys, aluminum
matrix composites, graphite, fiber reinforced plastics and other nonhomogeneous
materials, carbon-carbon composites, polyvinyl fluoride, fiberglass, Kevlar,
honeycomb materials(such as Nomex), Inconel, and copper alloys.
(ASM International.
Handbook Committee, Tool Materials,
pg. 98)
(Donald M. Mattox, Handbook of Physical Vapor Deposition (PVD)
Processing, pg.6)
My definition
is so long but it gives more detailed example for why it is used and why it is
important for machining processes.








hangi açıklamanın daha iyi olduğunu belirtebilir misin?
ReplyDeletekelimeleri düzenleyip eksikleri tamamlamak için perşembe gününe kadar vaktimiz var lisans öğrencileri olarak bugüne kadar bekleyip daha sonra değerlendirme yapmanız gerekiyor.
ReplyDelete